CHRONIC lung disease sufferers could be aided by a new drug which helps reduce the need for antibiotics, according to a clinical trial.
The final results from the Phase 2 WILLOW study were announced today by James Chalmers, British Lung Foundation Professor of Respiratory Research at the University of Dundee.
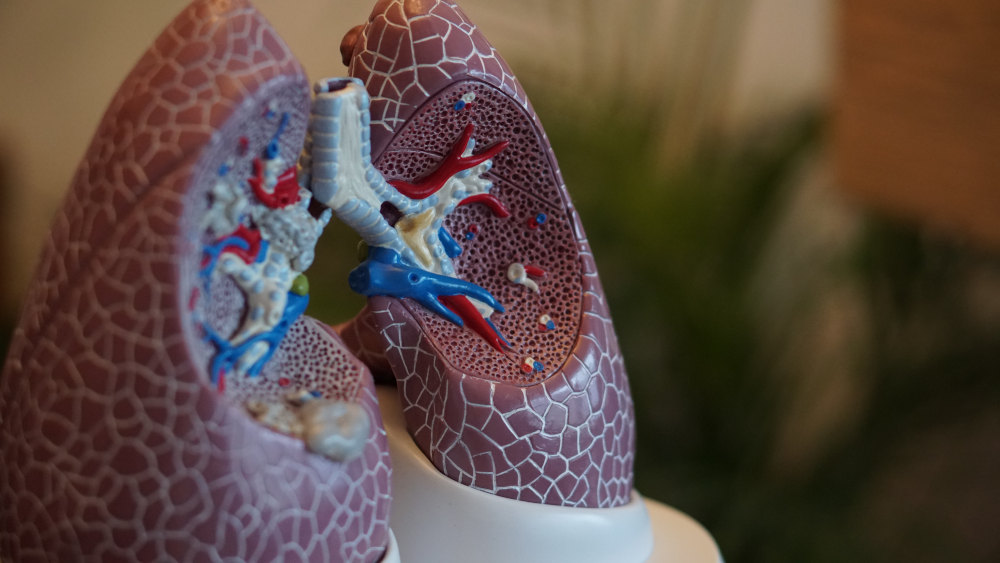
Scientists at the University, in collaboration with biopharmaceutical company Insmed Incorporated tested patients living with non-cystic fibrosis bronchiectasis, a long-term lung condition which causes abnormal widening of the airways.
The final results demonstrated that patients on the new drug, brensocatib, could potentially reduce their risk of pulmonary exacerbations by more than one-third compared to the placebo.
A further clinical trial, also led by the University’s School of Medicine, is currently exploring whether brensocatib can be used to prevent the need for mechanical ventilation in Covid-19 cases.
Bronchiectasis causes a build-up of excess mucus that can make the lungs more vulnerable to infection.
Complications associated with the disease, known as pulmonary exacerbations, often require emergency medical treatment.
Treatment for bronchiectasis is currently limited to controlling chest infection symptoms using antibiotics.
The drug will need to undergo further rounds of clinical trials and receive regulatory approval before it can be routinely prescribed by doctors, but the scientists have hailed these results as evidence that drugs can potentially be used to treat bronchiectasis directly.
Professor Chalmers said: “Living with bronchiectasis means that you’re constantly at risk of needing emergency treatment when the condition worsens.
“We currently rely on antibiotics to treat the chest infections caused by bronchiectasis, with few options to stop the disease from getting worse in the first place.
“We are excited by the possibility of a drug which can break the vicious cycle of inflammation, lung damage, and infection for these patients, giving them a much better quality of life.
“This trial is a world first in treating an incurable lung condition that affects millions of people worldwide.”
Martina Flammer, M.D., MBA, Chief Medical Officer of Insmed added:
“These findings are very meaningful for patients with bronchiectasis, who currently suffer from severe outcomes in the absence of an approved therapy.”
“Importantly, new data presented today demonstrate the relationship between neutrophil elastase reduction and the risk of exacerbation, and serve as further proof of concept of the potential of brensocatib and its unique mechanism of action.
“We look forward to initiating our Phase 3 program in bronchiectasis in the second half of this year, while also exploring the potential of brensocatib in other neutrophil-driven inflammatory conditions.”