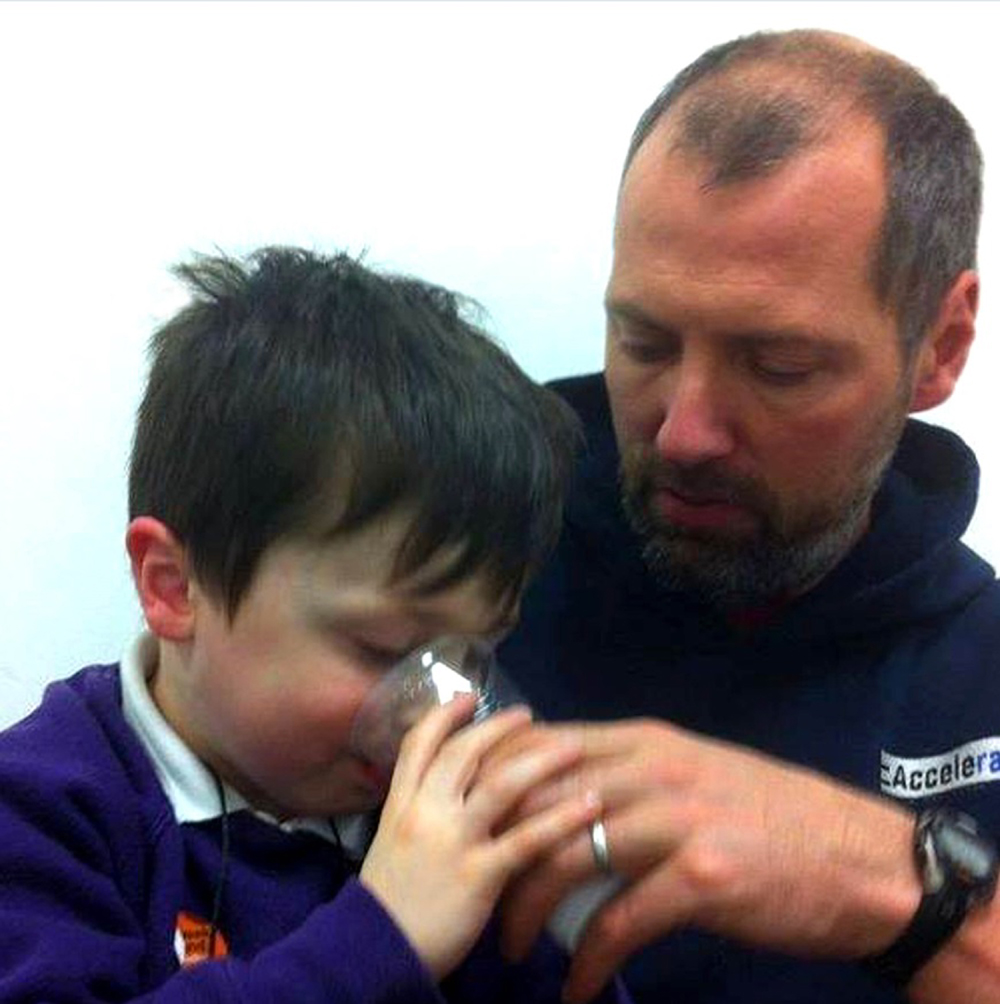
Five-year-old Cormac Fegan was born with Duchenne Muscular Dystrophy (DMD) – a life-shortening condition which causes muscle degeneration.
For the past four months he has been receiving Translarna, a new drug which has drastically improved his behaviour and physical strength.
But this week the Scottish Medicines Consortium (SMC) confirmed that it would not approve NHS funding for the treatment.
Now Cormac’s family have vowed to move to England – if the treatment is accepted south of the border.
The youngster, from Longniddry, East Lothian, frequently used to bite others and had trouble walking – common side effects of DMD.
Earlier this year NHS East Lothian agreed to fund his treatment for six months at a cost of £100,000, much to the delight of his parents.
His behaviour improved drastically and both his balanced and speed have increased over the months.But he will not be able to continue taking the life-changing medicine as the SMC was “unable to recommend ataluren (Translarna) for routine use”.
His parents are holding out hope than an appeal will overturn the decision, or that Translarna will become available on NHS boards in England and Wales following a National Institute for Health and Care Excellence announcement next week.
Cormac’s father, 43-year-old Gary, said: “I feel absolutely devastated at this decision, which at the end of the day came down to economics, not whether or not the treatment works.
“Two other families in Scotland have boys currently using Translarna and we know that it is effective and have tried to put this case across, which seems to have been dismissed.
“In the case of Cormac it has transformed his disruptive behaviour…as well as helping make him physically stronger.
“If it is approved in England we would definitely move, there’s no question. It would be the moral thing to do.”
He added: “The SMC has not grasped the importance of allowing these children to remain walking and independent.
“With life-expectancy of Duchenne children being just their mid-20s, and some children losing their lives long before that, you can well imagine the difference extra years walking – during a child’s most formative years – can make.
“This is children’s lives. It sickens me that it comes down to ‘what is the cost of the treatment?’ when the SMC haven’t truly understood the benefit.”
It is understood that US-based manufacturers PTC Therapeutics will be appealing the SMC decision.
Paul Lenihan MBE, CEO of charity Action Duchenne, said: “We are absolutely appalled by the SMC’s decision and method for evaluating this groundbreaking medicine.
“Despite doing everything we could to support this process…it is clear that Scotland has no framework for fairly appraising and funding drugs for rarer conditions.”
An SMC statement issued on Monday said: “The committee was unable to recommend ataluren (Translarna) for routine use in NHS Scotland for patients with Duchenne Muscular Dystrophy… as there was too much uncertainty about the overall clinical benefits it might bring in relation to its cost.”
Duchenne Muscular Dystrophy affects around 1 in 3,600 people and usually confines sufferers – most commonly boys – to a wheelchair by the age of 11 and leads to early death.
Translarna is the first treatment to protect boys from the most serious effects of the disease, and is currently available in several European countries including France, Italy and Spain.
Nine-year-old Michael Young, who also suffers from the disease, took part in a clinical trial of Translarna.
In January he travelled to Holyrood to meet Nicola Sturgeon and hand her a letter asking her to “help boys keep walking”.
Following the SMC decision, Michael’s mum has said they will launch an appeal to try and have it reversed.